Introduction
Growth hormone (GH) and insulin-like growth factor-1 (IGF-1) are important regulators of human skeletal development and maintenance from childhood to old age [1–5]. The somatotropic cells in the anterior pituitary gland are the source of GH secretion and they respond positively to GH-releasing hormone and ghrelin, and are inhibited by somatostatin [1, 6–9].
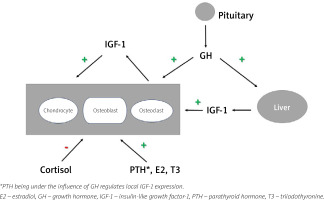
Growth hormone stimulates the expression of IGF-1 in the liver; it is subsequently secreted to the circulation and it enhances the production in numerous tissues of paracrine IGF-1, which acts locally. Growth hormone and IGF-1 regulate bone homeostasis. Growth hormone stimulates the maturation, proliferation and differentiation of chondrocytes and osteoblasts. Chondrocyte activation at the epiphyseal growth plates in children results in linear bone growth, while stimulated osteoblast activity increases bone formation. However, osteoclasts’ function is also induced, mostly by IGF-1 action, which induces synthesis of the receptor activator for nuclear factor κB ligand (RANKL), a crucial component of osteoclast formation and activation. Moreover, GH increases the production of osteoprotegerin, which serves as a soluble decoy receptor for RANKL, resulting in the downregulation of bone resorption.
Overall, the activated somatotropic axis increases bone formation and resorption, in consequence increasing bone turnover and remodeling, but bone formation prevails over resorption [1–6, 8].
Moreover, GH regulates calcium and phosphate homeostasis through stimulation of the 1α-hydroxylation of vitamin D, thus increasing calcium absorption and renal retention, and exerting antiphosphaturic effects.
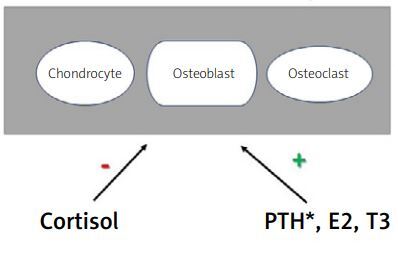
Other hormones may also play an indirect role in bone homeostasis through the somatotropic axis. Estrogens stimulate secretion of GH, although they inhibit IGF-1 production in the liver by antagonizing the GH receptor. However, they could stimulate paracrine IGF-1 secretion, similarly to thyroid hormones. On the other hand, androgens amplify GH action while glucocorticosteroids inhibit paracrine IGF-1 secretion [9–11] making that hormonal interplay rather complex (Fig. 1).
Eventually, growth hormone action leads to an increase in linear bone growth, accumulation of bone mineral content (BMC) and preservation of peak bone mass.
Growth hormone deficiency (GHD) may lead to growth impairment in children and prevent young adults from reaching peak bone mass. Due to reduced bone formation and loss of bone mineral density (BMD) it may increase the risk of osteoporosis. Thus, patients with GHD have increased bone fragility, resulting in increased morbidity and mortality. Clinical studies have shown that the prevalence of fractures in GHD adults is 2–7.4 times higher compared to age-matched controls [12].
Current GHD classification distinguishes patients with childhood-onset growth hormone deficiency (CO-GHD) and adult-onset growth hormone deficiency (AO-GHD) [1–3]. Various signs and symptoms of GHD in part depend on the age of the disease onset. Childhood-onset growth hormone deficiency subjects suffer from growth impairment, short stature, and craniofacial abnormalities, while AO-GHD patients experience mostly altered body composition, increased total fat, and reduced lean body and bone mass. Thus, in the long term adult GHD patients encounter increased risk of cardiovascular complications and fragility fractures, together with impaired quality of life [1–3, 12].
Methods
The literature search for articles published between January 2003 and December 2022 was performed using the PubMed database using the following queries: “dwarfism, pituitary” [MeSH Terms] OR “growth hormone deficiency” [Text Word]) AND “fractures, bone” [MeSH Terms] OR “bone density” [MeSH Terms] OR “muscle strength” [MeSH Terms].
We included full text clinical trials, meta-analyses and systematic reviews written in English, which concerned only human, adult subjects. Articles which were not directly relevant to our topic and studies involving small patient groups (less than 30) were rejected.
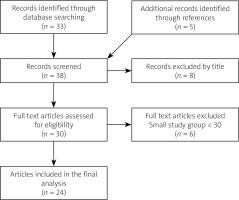
Finally, 24 articles were found eligible. Furthermore, the references of included studies were hand-searched in order to obtain a full understanding of the topic (Fig. 2).
Diagnosis of growth hormone deficiency
In clinical practice we may encounter patients with GHD presenting growth retardation, short stature, fractures, or osteoporosis. Growth hormone deficiency may have a congenital etiology arising from various hypothalamic-pituitary anomalies or more frequently in adults, it can be acquired [2].
Commonly recognized AO-GHD causes include pituitary tumors, mainly adenomas and surgical intervention or radiotherapy of the sellar region. The somatotropic axis is the most susceptible to radiotherapy of pituitary axes; hence GHD after irradiation is the first to develop and may occur as a sole deficit. However, frequently GHD coexists with other pituitary hormone deficiencies. It might be a result of hypophysitis either primary or secondary to systemic diseases such as sarcoidosis, histiocytosis or lupus erythematosus. Recently it become more frequent in patients receiving cancer immunotherapy, as a result of drug-induced hypophysitis [13].
Growth hormone deficiency may be seen in adults with a history of rhGH treatment during childhood.
The causes of GHD are shown in Table I.
Table I
Causes of growth hormone deficiency (GHD)
Although GHD symptoms are not as serious or life threatening as adrenal insufficiency or secondary hypothyroidism, it may also significantly impair quality of life and worsen the patient’s prognosis. Patients with GHD complain of weakness, malaise, and reduced energy level, even if other hormonal insufficiencies are adequately supplemented. On examination increased body fat, particularly visceral fat, increased skin fold thickness and epigastric fat, decreased muscle mass and muscle strength can be observed. Notably, the surplus of visceral fat mass is apparent even in individuals with normal body weight [14].
Skin tends to be pale, dry, and thin with visible wrinkles, especially on the forehead. Hair is thin, soft, and brittle. Sweating is significantly reduced due to the atrophy of the sweat glands. Growth hormone deficiency might also lead to an increase in total cholesterol, LDL cholesterol and apolipoprotein B. Patients with GHD are insulin resistant and often suffer from impaired glucose tolerance, probably associated with visceral fat accumulation [14, 15].
A patient presenting such symptoms suspected of GHD should have serum fasting IGF-1 measured. The results must be compared with the reference ranges adjusted for age and sex. Importantly, up to 20% of GHD patients may have IGF-1 in the lower limit of the normal range. After the exclusion of malnutrition, liver failure, decompensated diabetes, hypothyroidism or other catabolic states, low IGF-1 suggests GHD [1, 3].
Patients with high risk of GHD or with IGF-1 below the normal range should be referred to a reference center for dynamic hormonal testing. The adequate hormonal substitution of adrenal and thyroid axes must be implemented before testing, if necessary. Growth hormone stimulation tests such as the insulin tolerance test, tests with glucagon, growth hormone-releasing hormone (GHRH) and arginine are needed to confirm a diagnosis. However, if the patient has panhypopituitarism (deficiencies of ≥ three pituitary hormones) with a low IGF-1 level the tests can be omitted.
The transition period between the completion of bone linear growth and peak bone mass acquisition has been the focus of some studies [16–18]. The positive effect of GH on bone mineral density (BMD), bone mineral content (BMC) [7, 19–22] and fracture risk [23] has been confirmed.
For many years GH treatment was implemented in Poland only in the pediatric population due to the lack of funding in adult patients. In November 2020 the program of severe GHD treatment in adults was initiated in Poland. Thus, all patients with GHD suspected, e.g. those with a history of neurosurgical intervention, cranial irradiation or trauma and those treated with recombinant human growth hormone (rhGH) in childhood should be considered for rhGH treatment as adults.
Bone changes in growth hormone deficiency
Growth hormone deficiency causes a low bone turnover rate, which is reflected by decreased bone turnover markers of both formation, such as procollagen type I N-terminal propeptide, and resorption, such as C-terminal cross-linking telopeptide of type I collagen [5, 7, 18, 19, 24].
Bravenboer et al. [20] observed in histopathological image analysis that osteoid and mineralizing surface decreased and bone formation was generally slower in adult men with GHD compared to the control group. Growth hormone deficiency subjects had significantly more eroded bone surface [20]. Notably, GHD patients experience a mild state of PTH resistance, which resolves during rhGH treatment, as reported by Ahmad et al. [21].
Factors that negatively affect BMD in GHD are presumed to be: the young age of GHD onset, severe GHD and longer duration of untreated GHD [2, 3, 25]. Many researchers also found that hypogonadotropic hypogonadism with no adequate sex hormone replacement and over-treatment with glucocorticosteroids or thyroid hormones in hypopituitarism may reduce BMD. The same is true for early cessation of rhGH in children or young adults, before peak bone mass is reached [2, 3, 7, 25].
As mentioned, in children GH determines longitudinal bone growth and maturation. Bone mass is acquired throughout childhood and in adolescence. After a significant growth acceleration during puberty, which results from the combined influence of GH and sex hormones, peak bone mass is attained in the third decade of life [26].
Growth hormone deficiency leads to decreased BMD and BMC as measured by dual-energy X-ray absorptiometry (DXA) [2, 3, 27–29].
Childhood-onset growth hormone deficiency patients differ significantly from the AO-GHD group, mostly due to longer duration of deficiency and its influence on the transition period [30–32]. In CO-GHD the continuation of rhGH treatment for two years following longitudinal bone growth termination caused a prominent increase in BMD compared to untreated patients [17]. Patients who may not have achieved their potential peak bone volume can have an increase in bone volume during rhGH treatment. Furthermore, patients with CO-GHD are of shorter stature and have a notably smaller bone size and volume, which makes their BMD underestimated [26, 29, 33, 34]. Equally important is the fact that CO-GHD patients have decreased muscle mass and unfavorable body composition, which may also affect BMD measurements.
The most precise and reliable tool for assessing bone microstructure is high-resolution peripheral quantitative computed tomography (HR-pQCT) performed at the distal radius and tibia; however, it is not commonly available. Molitch et al. [3] reported that approximately 20% of AO-GHD and 35% of CO-GHD patients meet the densitometric criteria for osteoporosis (T-score ≤ –2.5).
The age of GHD onset is one of the determinants of osteopenia severity. Many studies have reported greater loss of bone mass and BMD in young adults, but not in patients over 55 years of age compared to the general population [3, 27, 33, 35].
Yang et al. [16] found that untreated young men with CO-GHD have compromised bone density, microarchitecture and resilience. Deficiency of GH in subjects with non-functioning pituitary adenoma was associated with a smaller decrease in BMD than in patients with a history of Cushing’s disease or acromegaly.
Of note, the prevalence of fractures in patients with isolated GHD is comparable to subjects with multiple pituitary hormone deficiencies, thus showing that GHD is the main risk factor of fractures in hypopituitarism [22].
Trabecular bone score (TBS) is another, more widely available method of assessing bone microarchitecture. Although TBS is a promising predictor of osteoporotic fracture risk, especially in endocrinopathies, the attempts to use that tool in GHD follow-up yielded equivocal results. Thus, other factors, such as bone turnover markers, may be a reasonable alternative. However, Kužma et al. [36] described a group of 147 patients with GHD who experienced a notable TBS improvement (4%) after 2 years of continuous rhGH replacement.
As mentioned above, GHD contributes to lower muscle mass and muscle strength, which increases the risk of falls and fragility fractures. Moreover, GHD secondary to central nervous system tumors or neurosurgical intervention can be accompanied by visual disturbances, visual loss or epilepsy. In consequence, it may also decrease physical activity and fitness, further decrease muscle strength and increase the risk of falls.
Impact of growth hormone therapy on bone turnover and bone mineral density
Recombinant hGH replacement leads to an increase in bone turnover, but the effect is biphasic. In the initial period of 6 to 12 months treatment results in a minute decrease in BMD [37–39].
This observation is consistent with the fact that in the initial treatment period bone resorption predominates. At first the rhGH effect on the skeleton leads to intense resorption, which predominates over the formation of bone tissue. In the course of the replacement therapy, an increase in calcemia and calciuria can be observed after 3 to 6 months.
This phenomenon probably results from the sensitization of receptors to PTH, which in turn leads to increased 1α-hydroxylation of vitamin D in the kidneys, decreasing calciuria in long-term follow-up [24, 40]. Moreover, rhGH exerts antiphosphaturic effects [41] and might re-establish the physiological PTH pulses, which tend to be disrupted in GHD [40]. After 12 to 24 months of rhGH replacement, bone formation begins to predominate and bone mass increases, which is reflected by the BMD results. The beneficial effects of rhGH replacement are seen earlier in children compared with adults (even after 6 to 12 months) [42–47].
The impact of prolonged therapy over a 10- and 15-year period have also been studied. Elbornsson et al. [45] observed that GH replacement for 15 years in GHD adults steadily increased the total body and lumbar spine BMC and BMD, but without a significant change in the femoral neck. A similar observation was made by Appelman-Dijkstra et al. [46] during a 15-year period. Most investigators agree that the increase in BMD is more pronounced at the lumbar spine compared to the femur and 18 to 24 months of rhGH replacement brings a 4–10% increase in BMD [42–47].
Of note, women responded to rhGH treatment with a smaller increase in BMD. This is probably due to estrogen-induced hepatic resistance to IGF-1 [2, 3]. Thus women need higher GH doses then men. Additionally, women on menopausal hormone therapy require higher GH doses when taking oral estrogen compared to those using transdermal systems [5]. Furthermore, the increase in BMD may persist even 18 months after discontinuation of treatment. One of the possible mechanisms underlying this effect is that GH facilitates bone remodeling, but is unnecessary for sustaining it. Nevertheless, the improvement in body composition fades as soon as the therapy is stopped [48, 49].
Bone mineral content rises more prominently than BMD due to increasing bone area by rhGH as shown by histomorphometric analysis [50–52]. In addition, recent meta-analyses indicate that sex, age, dosage and duration of treatment may influence the impact of rhGH on BMD [47, 53]. Thus, it seems that the reaction to rhGH is determined by multiple and diverse factors. Some studies have reported that low baseline BMD and IGF-1 were both predictors of positive BMD and BMC response during therapy [46, 51].
The use of high doses of rhGH in adults may cause a more prominent decrease in BMD in the initial phase, and some studies suggested that a lower replacement dose might be sufficient to facilitate a desirable result. However, the optimal dose is currently unknown and research data are ambiguous [54, 55].
The underlying cause of hypopituitarism may influence the impact of rhGH on bone mass. In patients with Cushing’s disease and prolactinomas the results of rhGH become noticeable only after a longer period of follow-up, compared with patients with non-functioning pituitary adenomas [56].
Effects of growth hormone on skeletal muscle mass and strength
Growth hormone and IGF-1 exert an anabolic effect on skeletal muscles resulting in increased skeletal muscle mass and strength [57, 58].
Growth hormone deficiency patients have significantly decreased muscle mass and strength, which improve after a period of at least 12 months of rhGH replacement therapy [59]. Moreover, impaired muscle strength might limit patients’ physical activity, which further worsens their muscle status. As a result, the risk of falls and fractures might be increased.
Effects of growth hormone replacement on fracture risk
Although BMD is a useful tool for predicting fractures in the general population, it is not ideal in GHD patients. Mazziotti et al. [24] observed that baseline BMD did not differ between subjects with incidents of vertebral fracture (VF) and those without. Notably, symptomatic VFs occur in over 30% of patients with AO-GHD, even those with normal BMD. Thus, the value of BMD in this context is limited and a history of VFs is the most important predictor of future osteoporotic fracture risk [60, 61].
Increased risk of clinical fractures was found in rhGH naïve GHD patients compared to treated ones, including a 7-fold fracture risk increase observed by Mazziotti et al. [62, 63]. Moreover, the risk of VFs in GHD may be increased by overtreatment of coexistent hypoadrenalism and hypothyroidism [2, 3, 63].
Holmer et al. [64] evaluated the prevalence of fractures in a large group of subjects on long-term rhGH therapy and compared them with matched controls. A high risk of fractures persisted in treated CO-GHD women, while in contrast, the risk in CO-GHD men and AO-GHD women improved and it did not differ from the general population [64]. This is possibly linked to sex hormone differences between CO-GHD men and women in the transition period.
The influence of GHD treatment on fractures and bone composition has been extensively investigated. Most studies consistently showed that rhGH therapy in GHD decreased the risk of fractures [22, 61, 64–67].
In a large cohort study, Mo et al. [61] reported markedly fewer fractures in the rhGH treated group compared to untreated GHD patients, both without previously reported osteoporosis. This suggests that starting rhGH replacement before the development of osteoporosis may preserve bone structure in adult GHD patients. However, this statement stands in contrast with previously mentioned authors who described that lower baseline BMD favors a more pronounced response to rhGH.
This discrepancy might be related to the severity of pre-existing osteoporosis, and the predictive role of BMD requires further research.
The drugs used in the treatment of osteoporosis are not well studied in the setting of GHD, and their safety and effectiveness in this context require further research. However, combining antiresorptive agents with rhGH in GHD patients seems to be a logical step in preventing bone loss and reducing VF risk [19]. Such an approach may be beneficial in subjects with significantly reduced BMD, which is consistent with the results of Biermasz et al. [68]. The duration and intensity of treatment are not well defined in patients with GHD.
Adverse effects of growth hormone treatment
Therapy with rhGH might lead to some adverse effects if the dose is too high, which is monitored by IGF-1 concentration during treatment. Water retention and the development of peripheral edema, paresthesia, myalgia or arthralgia are among the most common complications. Excessive growth hormone dosage increases insulin resistance and the risk of impaired glucose tolerance or even diabetes mellitus. Moreover, supplementary doses of hydrocortisone and levothyroxine in coexisting secondary adrenal insufficiency or hypothyroidism may need to be increased. Older patients treated with higher doses are reported to have greater risk of gynecomastia. Benign cranial hypertension and other complications are less common.
The risk of adverse events increases with age and warrants a more careful and slower dosage adjustment [1–3].
Conclusion
Growth hormone deficiency may affect both children and adults. It should be considered in patients with hypothalamic or pituitary lesions or anomalies, a history of neurosurgery, cranial radiotherapy, or traumatic brain injury, as well as those with other pituitary hormone deficiencies. Patients with untreated GHD have a lower bone turnover rate, leading to reduced BMD and increased risk of fractures. Thus, apart from treatment of coexisting hypogonadism, vitamin D and calcium supplementation, they should also receive rhGH replacement. Such substitution could be beneficial in patients with GHD, as it results in increased bone turnover, with bone formation predominance, increased BMD and reduced vertebral and non-vertebral fracture risk.
These beneficial effects are more prominent in male patients and possibly those with lower baseline BMD.
The indications for initiation anti-osteoporotic agents in patients with GHD in addition to rhGH treatment remain unresolved.
The limitations of our review include scarce data concerning long-term treatment in older populations and the limited number of subjects in the trials. Furthermore, most studies evaluating rhGH treatment lacked control groups and long-term follow-ups, which might make it difficult to reach accurate conclusions. Further research is required to fulfil current knowledge gaps.