Introduction
Idiopathic inflammatory myopathies (IIMs) are a group of autoimmune muscular diseases, which were categorized based on clinical manifestations by Bohan and Peter in 1975. The criteria of Bohan and Peter are the most widely used, but they have some limitations because each criterion is not defined precisely, especially based on new myositis-specific antibodies [1].
A review on IIMs by Simon et al. [2] in 2016 divided the disease into 5 subgroups: polymyositis (PM), dermatomyositis (DM), immune-mediated necrotizing myopathy (IMNM), sporadic inclusion body myositis (IBM), and anti-synthetase syndrome.
The European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria that were defined in 2017 for IIMs selected the most frequent IIM subgroups including DM, amyopathic dermatomyositis (ADM), juvenile dermatomyositis (JDM), IMNM, IBM, and PM for both adults and children for research studies and clinical trials [3].
There are other discriminations to find the patients with overlap myositis (OM) with DM features (OM) and pure DM like Senécal et al. [4] and Troyanov et al. [5].
Nailfold capillaroscopy (NFC) is both a clinical and a research tool, which helps clinicians in the evaluation of the microvasculature, and it is useful in differentiation of the underlying connective tissue diseases (CTD) and patients with Raynaud’s phenomenon [6]. It is well-known that patients with IIMS, especially DM, can have abnormal NFC results [7].
Despite numerous reports regarding capillaroscopy findings in systemic sclerosis (SSc), there are relatively few examinations in DM, especially for adult-onset DM [8] compared with JDM [8, 9].
In some articles using Bohan and Peter criteria, there was no significant relationship between NFC score and disease activity [10, 11], although some recent studies have shown the relationship of disease activity and capillaroscopy score and some improvements after treatment in adult patients with DM [12, 13].
There are some articles with limited numbers of patients for the evaluation of capillaroscopy in patients with OM [12, 13], and a few articles pulling the patients with OM from patients with PM/DM to find the differences between these patients in their clinical and capillaroscopy features [14, 15].
In this study, we evaluated the clinical, laboratory, and NFC changes in patients with IIMs using 2 different classifications, extracting the patients with OM from the new EULAR/ACR classification criteria and finding their manifestations.
We also evaluated the association between the activity of clinical and laboratory manifestations of the disease with capillaroscopy patterns and scores.
Material and methods
In this study, we selected 150 patients diagnosed with IIMs, including 113 females with a mean age of 36.28 ±14.82 years, and 37 males with a mean age of 33.97 ±20.94 years, who had referred from rheumatology clinics and in-hospital wards of Shiraz University of Medical Science including Motahary/Namazee, Faghihi, and Hafez for NFC from April 2010 to October 2018.
The patients who fulfilled the inclusion criteria were evaluated by the rheumatologist involved in this study using their referral data; the clinical examination on the day of capillaroscopy was done after the rheumatologist obtained the patient’s consent.
The study protocol was approved by the Human Ethics Review Committee of our University of Medical Science (no. IR.SUMS.MED.REC.1397.308).
Inclusion criteria
The patients were included in JDM if their age at presentation was < 18 years; then, they were considered as adult IIMs. The study samples were based on two different classifications:
New 2017 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria for adult and juvenile IM. We included the definite or probable (probability ≥ 55%) patients in our study [3]. In this group, we had a total of 150 patients and divided them into 4 sub-groups including DM – 81, ADM – 25, JDM – 25, and PM – 19.
Definition criteria of Senécal et al. [4] and Troyanov et al. [5] described patients with pure DM and OM with DM feature subgroups. The pure DM patients had DM rashes, proximal muscle weakness, no significant overlap clinical features, and no overlap autoantibodies. In this group, we extracted 104 patients including 53 (40 female and 13 male) patients with pure DM and 51 (45 female and 6 male) patients with OM from the first group of patients.
The patients with OM with DM features had proximal muscle weakness but no DM rash as the first manifestation of the disease, isolated heliotrope rash or Gottron papules, discrete and transient DM rashes, DM sine dermatitis, mechanic’s hands, palmar papules with or without ulceration, significant overlap clinical features, overlap autoantibodies, no DM specific autoantibodies, and no associated cancer within 3 years of diagnosis.
We defined the patients with pure PM if they were classified based on new EULAR/ACR classification without having any skin features of DM or autoantibodies for OM or any clinical manifestations of OM including retinitis pigmentosa (RP), arthritis, mechanic’s hands, interstitial lung disease (ILD), lower oesophageal dysmotility, SSc, systemic lupus erythematosus (SLE), or Sjögren’s syndrome features.
The patients with cancer-associated DM were not excluded from the study. According to the first criteria, we had the following groups: JDM, DM, PM, and ADM; then, we had 3 extracted subgroups: pure DM, pure PM, and OM with DM features.
In all patients, 8 nailfolds, except for 2 thumbs, were evaluated. The following clinical data were collected: age, gender, and disease duration (date of onset of the first muscle or skin symptom clearly related to DM). The patients were diagnosed as having ILD according to the results of chest computed tomography scan.
Exclusion criteria
We only had one diabetic patient, who was excluded from the study. Due to the low number of patients with IMNM and IBM, we excluded these 2 groups. Those without at least 2 good quality images from at least 6 fingers were excluded from the study. The patients with other known CTDs, who fulfilled their criteria including rheumatoid arthritis, primary Sjögren’s syndrome (pSS), SSc, and SLE, were excluded.
All clinical manifestations of the patients and their organ involvement were assessed. Laboratory test results including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), lactate dehydrogenase (LDH), creatine kinase (CK), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were extracted from the medical records at the time of evaluation. Anti-nuclear antibody (ANA) and extractable nuclear antigen (ENA) profiles, if available, were recorded.
The missed serologic data on the day of NFC was gathered from their admission charts available in hospital with their permission.
To assess the disease activity, the myositis disease activity assessment Visual Analogue Scale (VAS) portion of the myositis disease activity tool was used. We only used the VAS for global VAS score by physician, cutaneous VAS for DM and OM patients, and pulmonary VAS score for all patients [16].
The intensity of capillaroscopic changes ranged from 0 to 2:
score 0: normal (6–8 capillaries/mm2, hairpin-shaped loops arranged in parallel rows, absence of haemorrhages),
score 1: minor changes (6–8 capillaries/mm2, less than 50% tortuous loops, arranged in parallel rows, with no haemorrhages),
score 2: major changes (normal or decreased capillary density, more than 50% tortuous, enlarged loops, disarranged, with haemorrhages) [17].
The capillaroscopy parameters were evaluated by stereomicroscopy in power of × 250: distribution, morphology of capillaries, dimensions based on the largest diameter of the apical side (dilated > 20 μm, giant > 50 μm), capillary length (normal or elongated: ≥ 300 μm), mean capillary density (low density was defined as reduction of the normal number of the capillaries below 7 per linear millimetre), avascular area (intercapillary distance > 500 μm), and microhaemorrhages [13, 14], based on the last standardization of NFC and International Delphi consensus for reporting the data (2020).
The whole NFC findings were defined as: normal, scleroderma pattern, or non-specific abnormalities [18] and the international Delphi consensus for reporting the data [19].
For the number of haemorrhages in this study, we used the NEMO score, a cumulative number of micro-haemorrhages obtained from the 8 fingers of both hands, and then divided them into scores: zero = 0–1, few = 2–5, and multiple ≥ 6 [20].
For statistical analyses, if quantitative data had normal distribution, we used t-test or ANOVA to compare the means in different groups. If data did not have normal distribution, we used equivalent nonparametric tests.
We used the Pearson correlation coefficient to study the relationship between the 2 quantitative variables and the χ2 test to study the relationship between the 2 qualitative variables. Also, if we decided to categorize data according to a confounding variable and compared the 2 groups in different categories, we used analysis of variance. For all tests, a p-values < 0.05 was considered statistically significant.
Results
In the entire first classification study group, females dominated over the male subjects, 75.3% vs. 24.6% (p = 0.24). There was also a greater number of female patients in the subgroup analyses except in a subgroup of JDM patients. There was no significant difference in age between the male and female patients (p = 0.92). The characteristic clinical features and laboratory tests results are presented in Table I.
Table I
Clinical manifestations and laboratory tests in patients with idiopathic inflammatory myopathies in all patients and each sub-group including dermatomyositis, amyopathic dermatomyositis, juvenile dermatomyositis, and polymyositis, based on the first classification (new 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile inflammatory myopathies)
[i] ADM – amyopathic dermatomyositis, ALT – alanine aminotransferase, ANA – anti-nuclear antibody, AST – aspartate transaminase, CK – creatine kinase, CRP – C-reactive protein, DM – dermatomyositis, ESR – erythrocyte sedimentation rate, JDM – juvenile dermatomyositis, LDH – lactic acid dehydrogenase, PM – polymyositis, RNP – ribonucleoprotein, #due to low number of the data, p-value was not evaluable, ##(≥ 3 times above normal range).
In the first classification in the evaluation of clinical manifestations, except for unexplained fever (more in JDM) and polyarthralgia (more in ADM), the other clinical manifestations were observed more in DM patients (p-value < 0.05).
In the field of laboratory tests, the highest levels of CK (55%), LDH (57%), AST (56%), ALT (57%), ESR (57%), and CRP (58%) were seen in DM patients, and the lowest positive laboratory tests were found in ADM patients, except the lowest number of patients with high ESR and CRP, which were seen in the JDM group.
Anti-nuclear antibody was checked in 104 participants and was positive in 41 (40%) patients. It was positive in about half of the patients in all subgroups except for JDM, which was positive in only 6.7%. Anti-La, anti-Ro, anti-Jo-1, and RNP were mostly positive in the DM sub-group, but due to the low number of these data, the p-value was not evaluable between the subgroups. Moreover, positive anti-SC170 and anti-centromere tests were positive mostly in the ADM subgroup.
The dominant capillaroscopy pattern in all patients of the first classification was scleroderma pattern. The most dominant capillary changes were disturbed distribution (113 = 75.3%), haemorrhages (93 = 62%), and abnormal shapes (91 = 61%), and there was no significant difference among the subgroups (p-value > 0.05).
The dilated capillaries (77, 51.3%) and giant loops (68, 45.3%) were the next most dominant capillary changes in these patients, and they were not significantly different among the subgroups (p-value > 0.05). The low number of capillary (74 = 49.4%), elongation (70 = 46.6%), avascular area (49 = 32.6%), and then intravascular thrombosis (2 = 1.3%), and blood stasis (17 = 11.3%) were the next capillary changes that were seen in these patients, again with no significant difference among the sub-groups (p-value > 0.05), as shown in Figure 1 and Table II.
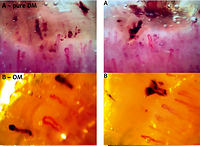
Fig. 1
Capillaroscopy of patients with active, pure dermatomyositis in power of 250× showing lowered density (6 capillary/mm), presence of giant loops, hairpin capillaries, and haemorrhages compatible with active scleroderma pattern of capillaroscopy (A), overlap myositis (anti-Jo-1 syndrome with dermatomyositis features) showing lowered density (6 capillary/mm), presence of giant loops, abnormal shape capillaries and haemorrhages compatible with active scleroderma pattern of capillaroscopy (B), juvenile dermatomyositis showing lowered density (5 capillary/mm), presence of giant loops, abnormal shape capillaries and haemorrhages compatible with active scleroderma pattern of capillaroscopy (C), pure polymyositis showing normal density (9 capillary/mm), normal diameter, hairpin capillaries and no haemorrhages compatible with normal capillaroscopy (D).
DM – dermatomyositis, JDM – juvenile dermatomyositis, OM – overlap myositi , PM – polymyositis.
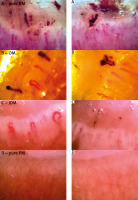
Table II
The capillaroscopy features, pattern, and score in patients with idiopathic inflammatory myopathies in all and each group including dermatomyositis, amyopathic dermatomyositis, juvenile dermatomyositis, and polymyositis, based on the first classification (new 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies)
The disturbed distribution had a significant association with the presence of Gottron sign (p-value 0.014) and heliotrope rash (p = 0.012). The presence of dilated and giant loop capillaries had an association with Gottron sign (p = 0.029). The presence of avascular area was correlated with Gottron sign (p-value 0.034) and the presence of multiple haemorrhages with Gottron sign (p-value 0.036), heliotrope rash (p-value 0.001), high CK (p-value 0.045), high AST (p-value 0.043), and high ALT (p-value 0.025).
Intravascular thrombosis was related to heliotrope rash (p-value 0.028), Raynaud’s phenomenon (p = 0.018), ILD (p = 0.021), and high ESR (p = 0.040). There was no relationship between other capillary components and clinical manifestations or laboratory tests (p-value > 0.05).
In the second classification, we had 104 patients including pure DM – 53 (40 female and 13 male) and OM – 51 (45 female and 6 male), with a mean age of 41.4 ±15.1 years for pure DM and 40.4 ±10.9 years for OM.
In the pure DM subgroup, the mean age was significantly higher than in the OM subgroup (p-value 0.007). In both subgroups, the female patients outnumbered the male ones (Table III).
Table III
Clinical manifestations and laboratory tests in patients with pure dermatomyositis and overlap myositis with dermatomyositis features in the second classification based on the criteria by Troyanov and Senécal
[i] ALT – alanine aminotransferase, ANA – anti-nuclear antibody, AST – aspartate transaminase, CK – creatine kinase, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, LDH – lactic acid dehydrogenase, OM – overlap myositis, Pure DM – pure dermatomyositis, RNP – ribonucleoprotein, #due to low number of this data, p value was not calculated, ##(≥ 3 times above normal range).
In the first classification, 28 (34.5%) patients with DM and 8 (42%) patients with PM were categorized as OM in the second classification. Therefore, after using the second classification, there were 11 patients with pure PM after extracting the OM patients from the PM group, including 7 females and 4 males with a mean age of 43.7 ±20.3 years.
Based on our second classification in clinical manifestations, Gottron sign (p = 0.029) and heliotrope rash (p = 0.024) were significantly more common in patients with pure DM.
Muscle weakness, V-sign, shawl sign, periorbital oedema, periungual erythema, unexplained fever, and dysphagia were also more prevalent in pure DM, but there was no significant difference between these groups (p-value > 0.05).
In laboratory tests, positive ANA was seen in 25 (60.9%) patients with OM, which was significantly higher than those with pure DM [12 (34.2%)] (p = 0.018). In addition, anti-La, anti-Ro, anti-SCL70, anti-Jo-1, and RNP were more positive in OM patients; however, due to the low number of these data, the p-value was not calculated.
The dominant (in > 70%) capillaroscopy pattern in the pure DM and OM subgroups was scleroderma pattern, with no significant difference (p-value of 0.452). The most dominant capillary changes were the disturbed distribution in both groups, and then haemorrhages and abnormal shapes, with no significant difference between groups (p-value > 0.05) (Table IV).
Table IV
Capillaroscopy features, pattern, and score in patients with pure dermatomyositis and overlap myositis in second classification based on the criteria by Troyanov and Senécal
In pure DM patients, there was a significant association between disturbed distribution with a high level of AST and ALT (p = 0.025, 0.021). The presence of giant loop capillaries was associated with a high level of ESR (p = 0.02). The presence of multiple haemorrhages was related to high levels of CPK (p = 0.008) and ALT (p = 0.025). The presence of elongated capillary loop was associated with ILD (p = 0.041).
There was no significant relationship between other components of NFC changes with clinical manifestations or laboratory tests (p-value > 0.05). In the OM subgroup, disturbed distribution was associated with muscle weakness (p-value 0.011) and Gottron sign (p-value 0.022), but between other components of capillary changes with clinical manifestation and laboratory tests there was no significant relationship (p-value > 0.05).
In pure PM patients, 7 (63.6%) had non-specific pattern of capillaroscopy, 3 (27%) had normal, and 1 (9%) had scleroderma pattern of capillaroscopy. The most dominant capillary changes were dilated capillary (6 = 54.5%), presence of disturbed distribution (4 = 36.3%), and elongation (4 = 36.3%).
Anti-nuclear antibody and anti-Jo-1 were checked only in 3 patients, and all of them were negative. In addition, biopsy was available in one (9%) patient that showed inflammatory myopathy with perivascular lymph infiltration.
Based on the first classification we saw a significant association between scleroderma pattern and high skin VAS score (p = 0.007). In addition, in the DM subgroup higher capillaroscopy score was related to high global and high skin VAS score (p-value 0.046 and 0.017, respectively).
There was a significant association between disturbed distribution, giant loops, low number of capillaries, avascular area, and high skin VAS score (p = 0.00, 0.001, 0.006, and 0.007, respectively). There was also an association between low number of capillaries and avascular area with high pulmonary VAS score (p = 0.042 and 0.006, respectively).
The presence of multiple haemorrhages also had a significant association with high global VAS score (p-value 0.00). Higher capillaroscopy score was associated with high skin VAS score (p-value 0.00), but we observed no significant relationship between other capillary components and myositis disease activity (p ≥ 0.05).
In the pure DM subgroup, disturbed distribution was associated with high global VAS score (p-value 0.037), multiple microhaemorrhages with high global (p-value 0.001) and high skin VAS score (p = 0.001). Also, there was a significant relationship between capillaroscopy score 2 and high global VAS score (p-value 0.047), and the relationship between capillaroscopy score 2 and high skin VAS score was almost significant (p-value 0.050).
In the OM patients, scleroderma pattern of capillaroscopy was associated with high global and high skin VAS score (p-value 0.027 and 0.008, respectively). The presence of abnormal shape, giant loops, avascular area, or haemorrhages was associated with high skin VAS score (p-value 0.046, 0.042, 0.039, and 0.027, respectively).
Discussion
In this study, we described the clinical manifestations, laboratory tests, and NFC features in patients with IIMs using 2017 EULAR/ACR classification criteria; then, we extracted 3 groups of pure DM, pure PM, and OM and re-evaluated them. In both classifications, the dominant capillaroscopy pattern was scleroderma pattern in all sub-groups except for pure PM. In the DM and pure DM sub-group, a significant association of higher capillaroscopy score with high global and high skin VAS score was detected. In OM patients, we saw a significant association of the presence of scleroderma pattern with high global and skin VAS score. We showed that 15.6% of the patients with OM were named PM in the first classification.
In our patients Gottron sign and heliotrope rash were seen more in patients with pure DM than the OM group. In a previous study [5], similarly to our findings, heliotrope rash, Gottron papules, V-sign, shawl sign, and periungual erythema were more seen in pure DM than OM with DM features, whereas mechanic hand was present only in OM patients. Nuńo-Nuńo et al. [14] in a study on 324 patients with OM, PM, and DM showed more arthritis, puffy fingers, sclerodactyly, dysphagia, RP, leucopaenia, thrombocytopaenia, ILD, and renal manifestations in the OM group.
In the field of laboratory tests, the lowest increase in ESR and CRP was seen in JDM patients. The high levels of CK, LDH, AST, ALT, ESR, and CRP were not different between the pure DM and OM subgroups. In a study by Troyanov et al. [5], a high level of CK was seen more in OM patients.
Antinuclear antibody was positive in 41 (40%) patients in our study. It was positive in about half of the patients in all subgroups, except for the JDM subgroup, which was positive in only 6.7%. Positive ANA was observed more in OM patients than in pure DM patients. Similarly to our findings, in a previous study [21] on 53 patients with DM and PM based on the criteria of Bohan and Peter, ANA was positive in nearly half of the patients.
In the first classification, the ENA tests including anti LA, anti-Ro, anti-Jo-1, and RNP were mostly positive in the DM subgroup. According to the second classification, the ENA tests including anti-La, anti-Ro, anti-SCL70, anti-Jo-1, and RNP were more positive in OM patients. Similarly to our findings, in Troyanov’s study [5], anti-Ro, anti-Jo-1, and RNP were observed more in the OM group.
In the presented study the dominant capillaroscopy pattern in DM, ADM, and JDM subgroups was scleroderma pattern. The dominant capillaroscopy pattern in the pure DM and OM subgroups was also scleroderma pattern, which was not significantly different among these groups. In the DM, pure DM, and nearly all the OM patients, there was an abnormal pattern of capillaroscopy. In pure PM patients, the scleroderma pattern was found only in 1 (9%) of them, although scleroderma pattern was seen in 42% of the PM group before extraction of the pure PM subgroup.
In a previous study on 27 DM patients based on Bohan and Peter classification criteria [11], similarly to our findings, the dominant capillaroscopy pattern was scleroderma pattern, which presented in 88.9% of their patients. In another investigation on 50 DM patients [22], scleroderma pattern was shown in 74% of their patients. In one study on JDM patients [23], scleroderma pattern was observed in most (84%) of the patients.
In the evaluation of the component of capillary changes, the dominant capillary changes were disturbed distribution, microhaemorrhages, and abnormal shapes in the DM, ADM, JDM, pure DM, and OM subgroups. In line with our study, in a study by Selva-O’Callaghan et al. [10], the disturbed distribution and then abnormal morphology were the most capillary changes, and these changes were seen more in the DM subgroup. In a study on 27 DM patients, again, the abnormal shapes, disturbed distribution, abnormal dimension, and microhaemorrhages were the dominant capillary changes [11].
In the first classification, we found that the presence of disturbed distribution, dilated and giant loop capillaries and avascular area had a significant association with skin manifestations. The presence of multiple haemorrhages had a significant relationship with skin rash, high CK, AST, and ALT.
Intravascular thrombosis was related to heliotrope rash, RP, ILD, and high ESR in all patients. In the pure DM subgroup, there was a significant association between disturbed distribution and high levels of AST and ALT. The giant loop capillary was associated with high levels of ESR. The presence of multiple haemorrhages was related to high levels of CPK and ALT.
In addition, the elongated capillary loop was associated with ILD. In the OM subgroup, the presence of disturbed distribution was associated with muscle weakness and Gottron sign. In one study on 53 patients with DM and PM according to Bohan and Peter criteria, the presence of higher numbers of capillaroscopy alterations and microhaemorrhages had a significant association with RP [10].
In addition, in a study it was shown that the presence of more microhaemorrhages, capillary disorganization, and abnormal shapes correlated with poor prognosis and higher lung fibrosis in patients with ILD along with DM [24].
We showed that 42% of patients with PM could be categorized as OM. According to the first classification, 42% of the patients with PM had scleroderma pattern of capillaroscopy, whereas in pure PM patients, scleroderma pattern of capillaroscopy was only found in 1 (9%) of them. Therefore, it seems that extracting the patients with OM from the first classification using NFC could be helpful in future studies.
In a previous article [25], on 70 patients with IIMs, scleroderma spectrum abnormality was found in 27.8% of the PM patients or in a study by Manfredi et al. [26] and ANA was positive in 65% of PM patients; the reason for this finding might be that the OM was not included in their classification; we found scleroderma pattern in only one patient with pure PM, and ANA was negative in all of them.
In this study, we found a significant association between scleroderma pattern in NFC and high skin VAS score based on the first classification. In OM patients, there was a significant association between scleroderma pattern of capillaroscopy and high global and skin VAS score.
There was also a significant association between the presence of disturbed distribution, giant loops, low number of capillaries, avascular area, and high skin VAS score. There was also an association between low number of capillaries and high pulmonary VAS score. The presence of avascular area and multiple haemorrhages was associated with high global VAS score.
In the pure DM subgroup, the presence of disturbed distribution was associated with high global VAS score. Multiple haemorrhages were related to high global and high skin VAS score. In addition, higher capillaroscopy score had a significant association with high global VAS score. In the OM subgroup, the presence of abnormal shape, giant loop capillaries, avascular area, and multiple haemorrhages was associated with high skin VAS score.
A previous study on DM patients showed that loss of capillaries was associated with muscle and global disease activity, and high scores of haemorrhage had a significant association with skin disease activity [22].
Recent studies also showed that there are inverse correlations between NVC scores and disease activity before treatment (n = 10) and that the density can be a dynamic marker of global disease activity in adult patients (n = 15) with DM that can be improved after treatment [12, 13].
To summarize, the presented study describes the clinical manifestations, laboratory tests, and NFC features in patients with IIMs (2017 EULAR/ACR classification criteria) as well as a comparative analysis of 3 subgroups (pure DM, pure PM, and OM). In both classifications, the dominant capillaroscopy pattern was scleroderma pattern in all subgroups except for pure PM, and the most dominant capillary changes were the presence of disturbed distribution, abnormal shapes, and haemorrhages.
In addition, we found an association between some abnormal components of capillaroscopy and clinical manifestations/laboratory tests. In the PM and pure DM subgroups, a significant association between higher capillaroscopy score with high global and high skin VAS score was detected. In OM patients, we saw a significant association between the presence of scleroderma pattern and high global and skin VAS score. We showed that 15.6% of the patients with OM had established PM as a first diagnosis (first classification), which was re-evaluated. In the second classification, Gottron sign and heliotrope rash were seen more often in patients with pure DM. Troyanov et al. [5] presented similar observations, i.e. that heliotrope rash, Gottron papules, V-sign, shawl sign, and periungual erythema were more likely to be seen in pure DM than OM with DM features, whereas mechanic hand was present only in OM patients.
Study limitations
Our study had some limitations. Muscle biopsy was done in only 7% of our patients, and 27% of all patients had probable IIMs based on the first classification. Another limitation was that muscle-specific antibodies (MSA) were not assessed. Based on our study, nearly all patients with DM, pure DM, and OM did not have a normal capillaroscopy, and our pure PM patients mostly had normal or nonspecific abnormalities.
Therefore, it would be better to include capillaroscopy in IIM classification scores to increase the sensitivity of this classification, especially extracting the patients with OM and pure PM and when high specific myositis-specific autoantibodies and/or muscle biopsy are not available or not helpful for exact diagnosis. This assessment may also be helpful as a predictor of response to treatment.
Conclusions
In this study, in patients with DM, pure DM, ADM, JDM, and OM, the dominant capillaroscopy pattern was scleroderma pattern, disturbed distribution, microhaemorrhages, and abnormal shapes, but in the pure PM sub-group, the dominant pattern was non-specific abnormalities and normal NFC.
In the DM patients, we found a significant association between global and skin VAS disease activity and higher capillaroscopy score. Some of our patients with PM in the first classification could be categorized as OM.
Therefore, adding abnormal capillaroscopy components like haemorrhages, disturbed distribution, and abnormal shapes in future classifications can help us in more detailed classification of patients.